About MVPE-CC Project
January 9, 2019 | More About MVPE-CC | Reading time: 6 min
Background & Strategy
1.Malaria Vaccine Pilot Evaluation-Case Control project brings together a multidisciplinary consortium by leading African clinical trial centres in partnership with European and global institutions
with complementary expertise and proven track record from the following institutions: Kintampo Health Research Centre (KHRC), Ghana, kintampo-hrc.org.
2.European Vaccine Initiative EWIV (EVI), Germany, euvaccine.eu.
3.London School of Hygiene and Tropical Medicine (LSHTM), United Kingdom, lshtm.ac.uk
4.African Research Collaboration for Health Limited,
Kenya, aphrc.org.
5Kenya Medical Research Institute (KEMRI), Kenya, kemri.org.
6.University of Malawi, College of Medicine Malawi,
Malawi, medcol.mw.
7.PATH, United States, path.org.
Malaria remains a major killer of children in Africa, where over 90% of cases occur. The RTS,S malaria vaccine was shown in a large phase-3 trial to be efficacious, with an acceptable safety profile. Modelling estimates indicate that its addition to current malaria-control measures could save tens of thousands of lives. Its wide implementation is delayed by a lack of data on effectiveness and safety when delivered through the national Expanded Programs on Immunization (EPI). The World Health Organization (WHO) recommended pilot introduction to evaluate feasibility, impact and safety, with emphasis on the safety signals observed in the phase-3 trial, in routine use to inform a global policy recommendation. Through the WHO-coordinated Malaria Vaccine Implementation Programme (MVIP), pilot implementation of RTS,S is well underway in three African countries, with more than 700,000 children vaccinated.
Independent of vaccine implementation is the ongoing evaluation to measure feasibility of reaching children with the recommended four-dose schedule requiring immunization contact and vaccine impact and safety in routine use. The evaluation component of the MVIP is referred to as the Malaria Vaccine Programme Evaluation (MVPE) (https://www.who.int/initiatives/malaria-vaccine-implementation-programme).
The surveillance established for the MVIP provides an opportunity for case-control studies to be conducted to address additional questions about vaccine impact, that cannot be answered directly through the cluster-randomized design.
Additionally, new data bring into question the importance of the 4th dose in the recommended schedule. The MVIP Programme Advisory Group recommended case-control studies, as a priority, to address these questions to inform policy.
We propose to embed case-control studies into the Malaria Vaccine Pilot Evaluation (MVPE), to strengthen the evaluation of safety and effectiveness (including the incremental effectiveness of a 4-dose vs 3-dose regimen), and to develop
tools that can be used by national immunization and malaria control programmes to monitor vaccine effectiveness following introduction.
The case-control studies will take advantage of the community-based mortality surveillance and hospital-based disease surveillance systems that have been established as part of the MVPE. We will estimate excess risk of meningitis and cerebral malaria and of mortality in girls, associated with RTS,S vaccination, three safety signals observed in the phase-3 trial, and will estimate vaccine effectiveness in preventing malaria in children, the importance of rebound effects, and the incremental benefit of receiving the 4th dose. The project will make important contributions to policy recommendations about the wider use of the RTS,S malaria vaccine at the global and country-level and help maximize acceptability, uptake and impact if recommended for use. The project also aims to promote the use of case-control approaches by EPI and malaria control programmes in high-burden countries, strengthening their capacity to monitor the effectiveness of the malaria vaccine. A strong dissemination and communication plan is proposed to maximise the potential impact of this study.
Incidence-density case-control designs will be used with community controls recruited concurrently with cases, matched on date of birth and neighbourhood. This type of design allows the incidence rate ratio associated with the exposure (RTS,S vaccination) to be estimated. The same methods will be used in each country, and the studies are powered for pooled analyses across the three countries.
Results from the case-control studies within the broad Malaria Vaccine Pilot Evaluation will help to inform global vaccine recommendation decisions on the potential use of the malaria vaccine in sub-Saharan Africa. The studies will estimate excess risk of meningitis and cerebral malaria, and of mortality in girls, associated with RTS,S vaccination. In addition to these safety signals observed as part of the phase-3 trial, the studies will estimate vaccine effectiveness in preventing malaria in children, the importance of rebound effects, and the incremental benefit of receiving the 4th dose. The project also aims to promote the use of case-control approaches by the Expanded Programme of Immunization (EPI) and malaria control programmes in high-burden countries, strengthening their capacity to monitor the safety and effectiveness of malaria vaccines.
Organisational Structure
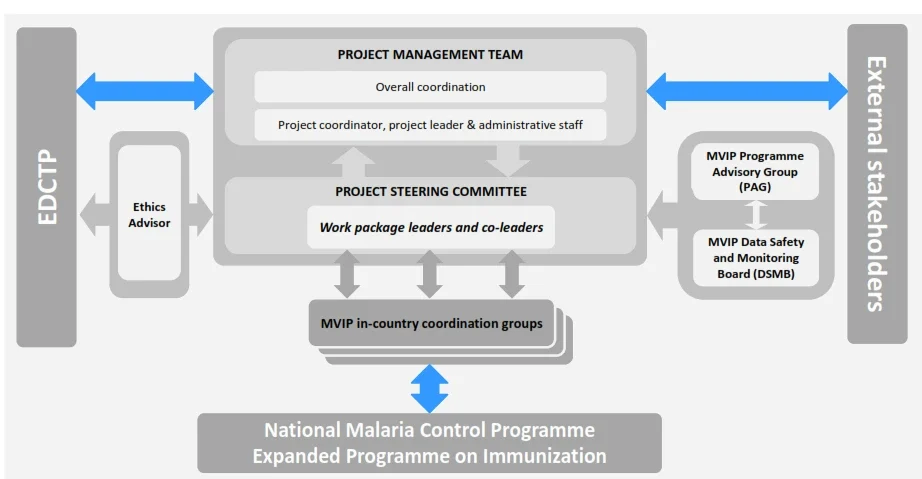
Work packages
WP1 Title: Implementation of clinical case control study embedded in MVPE (Clinical outcome – Meningitis, severe malaria, cerebral malaria) The objectives of work package 1 are to obtain ethics and regulatory approvals in Ghana, Kenya and Malawi; develop standard operating procedures, and recruit and follow up all study participants for the meningitis, severe malaria and cerebral malaria case control study
WP2 Title: Implementation of mortality case control study embedded in MVPE
WP3 Title: Development of study protocols, standard operating procedures, and data management tools, and statistical analyses Work package 3 seeks to contribute to development of protocol and SOPs ensuring use of rigorous, best practice methodology; develop and implement a data management plan; develop and implement a statistical analysis plan, and to develop a toolkit of case-control methodology in assessing vaccine safety and effectiveness
WP4 Title: Capacity building among researchers and program managers of Ministries of Health Objectives of work package 4 are to 1) conduct 3 webinars on a) case-control methodology, b) data analysis of case-control studies, and c) reporting of case-control studies with special focus on safety and effectiveness during scale up of malaria vaccines; and 2) conduct on-site training with practical sessions for MoH from target countries who will travel to the pilot countries during the conduct of the studies in Ghana, Kenya and Malawi
WP5 Title: Community engagement and dissemination of lessons learned from case-control studies, including policy implications, and leveraged by MVIP communications funded through the larger programme Work package 5 seeks to create awareness of the goals, methodologies, and outcomes of the case-control studies, highlighting implications for policymaking; identify key stakeholder groups at national and subnational levels; create opportunities for stakeholders at multiple levels to understand and engage with the evidence generated by the case-control studies in the context of findings of the wider pilot evaluation. Additionally, we will facilitate sharing of messages and information related to the studies’ goals, methodologies, and outcomes among project partners in the participating countries in the interest of aligning communications and building internal project cohesion, and, translation of evidence from the scientific research into policy-relevant tools and materials, to support policymaking and broader efforts to save lives from malaria.
WP6 Title: Project management and coordination Objectives of work package 6 are to;
1) set-up an efficient management infrastructure including management bodies, independent scientific and ethics advisory committee,
data safety and monitoring board, and management procedures and tools.
2) ensure observance of the required ethical standards.
3) conduct contractual management of the consortium.
4) implement day-to-day operational
project management and,
5) strengthen project management capacities.
Partners / Collaborators
European Vaccine Initiative
About EVI: European Vaccine Initiative (EVI) is a not-for-profit Product Development Partnership (PDP) that supports the development of safe, effective and affordable vaccines for global health through collaboration
and coordination. EVI operates as an independent, science-driven organisation, leading innovative solutions in vaccines through research, development, capacity strengthening and advocacy. EVI works closely with partners and
donors worldwide to move vaccines forward. EVI´s portfolio and activities are diverse. Since its inception in 1998, EVI has collaborated in the development of more than 40 vaccine formulations against diseases like malaria,
leishmaniasis, Zika virus and diarrhoeal diseases, 9 of which progressed into mid-stage clinical development. EVI is also supporting vaccine development through cross-cutting projects ranging from the development of non-animal
approaches for vaccine quality control testing and assay harmonisation, to the establishment of a sustainable European vaccine infrastructure that can accelerate vaccine development across several disease areas, among many
other projects and activities.
Kintampo Health Research Centre
About KHRC: Kintampo Health Research Centre is one of the leading research institutions in Ghana, established in 1994 as part of the Ghana Health Service Research and Development Division. KHRC has well-equipped
clinical trial facilities, clinical laboratory with international quality-assurance systems, data management centre capable of processing/protecting large clinical datasets. Since 1995 KHRC has engaged community members
to guarantee culturally acceptable research practices using consensus-building techniques in planning and implementation. With >60 scientific staff of various research disciplines, KHRC conducted clinical trials leading
to high public health impact. KHRC is currently the coordinating institution for Malaria Vaccine Pilot Evaluation in Ghana. KHRC operates with support from generous donors, funding agencies, collaborators, Ghana Health
Service and the Ministry of Health.
London School of Hygiene and Tropical Medicine
About LSHTM:London School of Hygiene and Tropical Medicine is a world-leading centre for research and postgraduate education in public and global health. LSHTM delivers research-led educational programmes to future
health leaders, managers and researchers across the world with 4,200 Master’s and Doctoral students, 1,000 students each year on short-courses, and 70,000 using free online-courses. LSHTM staff have expertise in vaccine
evaluation, including extensive experience in the design, conduct and analysis of case-control studies. LSHTM is already involved in MVPE, providing data management/statistical support.
African Research Collaboration for Health Limited Kenya
About ARCH:University of Oxford, Wellcome Trust and KEMRI formed the KEMRI-Wellcome Trust Research Programme (KWTRP) in 1989 with the vision/mission to deliver high quality research that is relevant to global health
and to build local capacity for undertaking research. KWTRP’s research portfolio includes implementation science, epidemiology, clinical trials, molecular biology and social science. Vaccine development is one of five major
research themes, with e.g. Phase II/III RTS,S vaccine trials performed in collaboration with KEMRI-CDC. KWTRP members serve on the Kenyan technical advisory group for vaccination policy for the MoH, and on numerous other
policy interfaces. KWTRP established standardized enhanced clinical surveillance, in partnership with the MoH, and six hospitals are sentinels for MVIP evaluation.
Kenya Medical Research Institute (KEMRI)
About KEMRI-CGHR:Kenya Medical Research Institute (KEMRI) is a state corporation with the mission to improve human health and quality of life through research, capacity building, innovation and service delivery.
The Centre for Global Health Research (CGHR) is located in Kisumu, western Kenya, with high burden of malaria/other infectious diseases and a high infant/under 5 child mortality. Since its inception (1984), CGHR performed
ground-breaking research on epidemiology, surveillance, clinical trials, implementation research, qualitative research on infectious diseases of public health importance including malaria. KEMRI-CGHR has excellent capacity:
well-trained staff, state-of-the-art laboratories, financial/administrative support systems, good information communication technology and data management systems.
College of Medicine, University of Malawi
About COM:College of Medicine, University of Malawi is the only medical college in Malawi with mandate to train health workers and generate research data for policy. COM collaborated with Malawi national health programs
to become a center of excellence in training/research. COM is leading a research consortium on MVPE in eleven districts across Malawi. COM has all required facilities: training facilities, secure internet connection, robust
Data Management and Biostatistics Unit, modern laboratories and research support center to manage research grants. Senior staff sits on several technical working groups where they influence both malaria and vaccine policy.
PATH
About PATH: PATH is a global non-profit organization that works to accelerate health equity by bringing together public institutions, businesses, social enterprises, and investors to solve the world’s most pressing
health challenges. PATH’s Center for Vaccine Innovation and Access accelerates the development and delivery of life-saving vaccines around the world and partners with countries in sub-Saharan African and Southeast Asia
to advance and sustain immunization equity and vaccination coverage. PATH has two decades of experience in advancing various malaria vaccine candidates, including RTS,S, and is now partnering to support the pilot introductions
of the vaccine.
